As lithium-ion battery materials evolve, suppliers face new challenges
Lithium-ion batteries (LIBs) began to impact the rechargeable or secondary battery market for portable electronic devices at the turn of this century. Nickel metal hydride (NiMH) rare earth-containing batteries, the main competitor two decades ago, quickly lost share to LIBs due to the combination of superior rechargeabil- ity, higher energy density, and lower size and weight.
All LIBs have one element in common, lithium. Yet the
combination of cathode materials, the beating heart of the battery
cell, is in constant flux. Lithium cobalt oxide (LCO) cathodes,
which have a 60% cobalt content and are installed in the bulk of
smart phones and laptops, are steadily losing market share to
higher energy density lithium nickel manganese cobalt oxide (NMC)
cathodes. NMC cathodes have a lower cobalt content, less than 20%,
and they power high-performance long-range electric vehicles
(EVs).
NMC technology is experiencing a noticeable shift from cobalt-rich
to nickel-rich cathodes, as seen in the Figure below.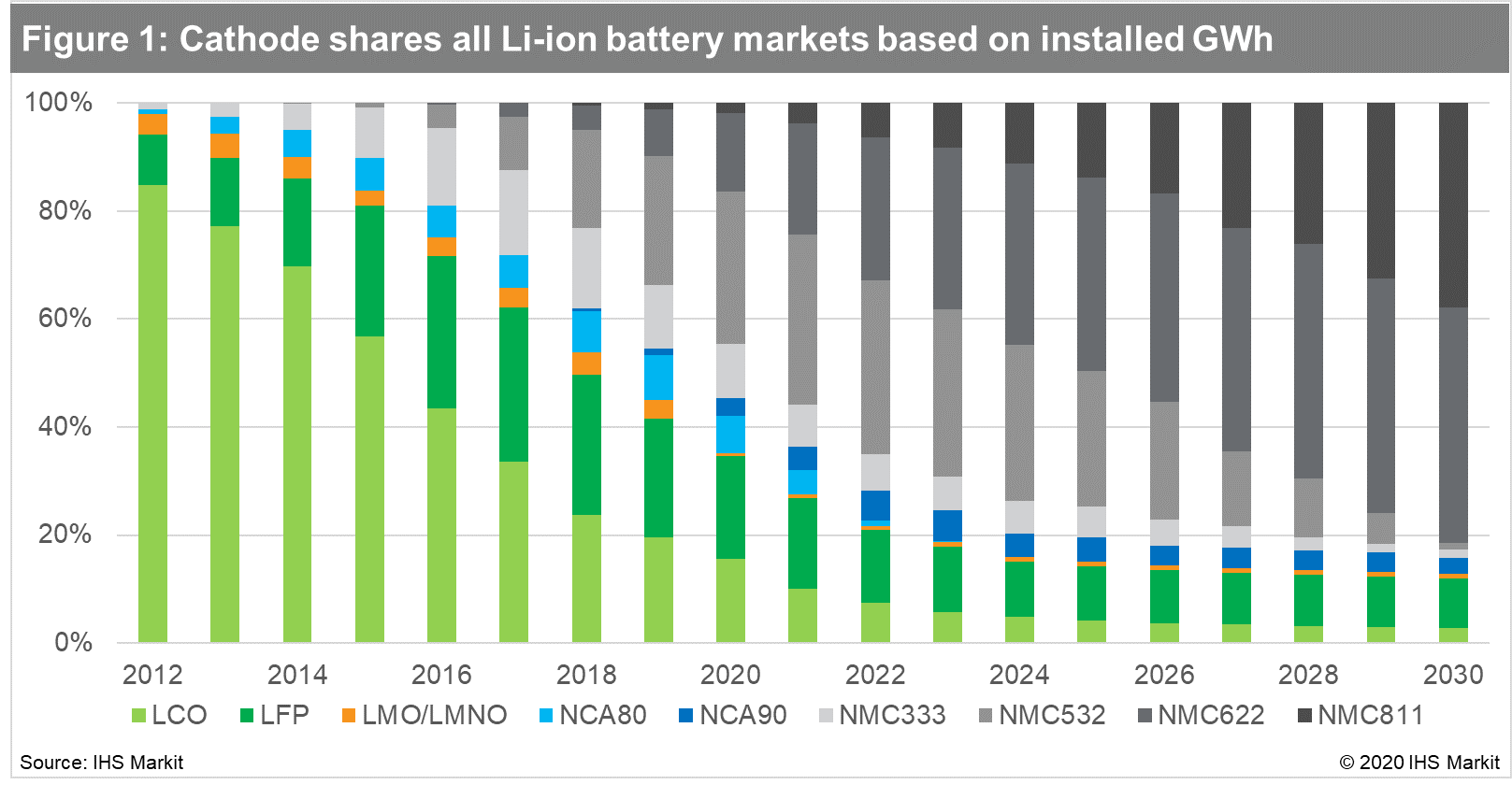
In general, a higher cobalt content correlates with a more
expensive battery, and higher nickel content equals greater energy
density. Energy density is proportional to the range that the EV
can drive on a single charging cycle. Lithium iron phosphate (LFP)
and lithium manganese oxide (LMO) began to be phased out of the
market because of low energy density. LFP, however, remains in the
running thanks to its inherent safety, low cost, and independence
of the cobalt and nickel supply chain. IHS Markit is forecasting a
resurgence in LFP in EV's, whilst also continuing to gain market
share in e-buses, and grid storage applications. Lithium nickel
cobalt aluminum oxide (NCA) cathodes, used by Tesla, are forecast
to lose share over the next five years.
Will nickel challenge cobalt's crown?
Probably yes, but not so fast. Today, LIBs count for over
half of global cobalt consumption. Despite the migration towards
high-nickel cathodes, total cobalt demand is expected to double by
2025, with demand driven by EVs. Cobalt is necessary for the
structural stability of the battery. Lack of structural stability
results in decreasing cycle life and a sharp loss in storage
capacity.
Is cobalt the blood diamonds of
batteries?
The sources of cobalt include: large-scale mines (78%), artisanal
and small-scale mining (12%), and recycling (10%). The use of
cobalt is associated with considerable reputational risk for the
automotive and electronics industries. Large-scale mines frequently
rely on dubious practices around acquiring and maintaining mining
licenses. Artisanal and small-scale mines are sometimes illegal.
They often use irresponsible practices and inadequate equipment,
putting the health of workers at risk. About 2% of the total cobalt
supply is mined by child labor.
The supply of mined raw materials is highly concen- trated in the
Democratic Republic of Congo, which poses a risk to the robustness
of the entire supply chain. Approximately 99% of cobalt is
extracted as a byproduct of copper and nickel mining, which makes
it greatly dependent on future supply and demand developments of
those materials. The processing of raw materials into cathodes,
cells, and batteries is concentrated in Asia, particularly mainland
China - which also poses a risk to the security of the supply
chain.
By 2030, over 80% of lithium will be mined for
EVs
In 2000, about 9% of lithium produced was used for batteries. By
2020, this share rose to 66% and it is forecast to reach over 90%
by 2030. Other lithium applications - including ceramics, glass,
and lubricants - still play an important role, but they tend to
grow along with GDP. Lithium raw materials and chemicals suppliers
had to respond quickly to produce different high-specification
materials and production quantities. While demand for lithium and
cobalt is already dependent on the battery market, less than 5% of
nickel is consumed in LIB manufacturing. Most nickel is consumed in
the manufacture of stainless steel. As shown in Figure below, the
growth of cobalt in LIBs will begin to slow, whereas nickel
consumption will increase significantly.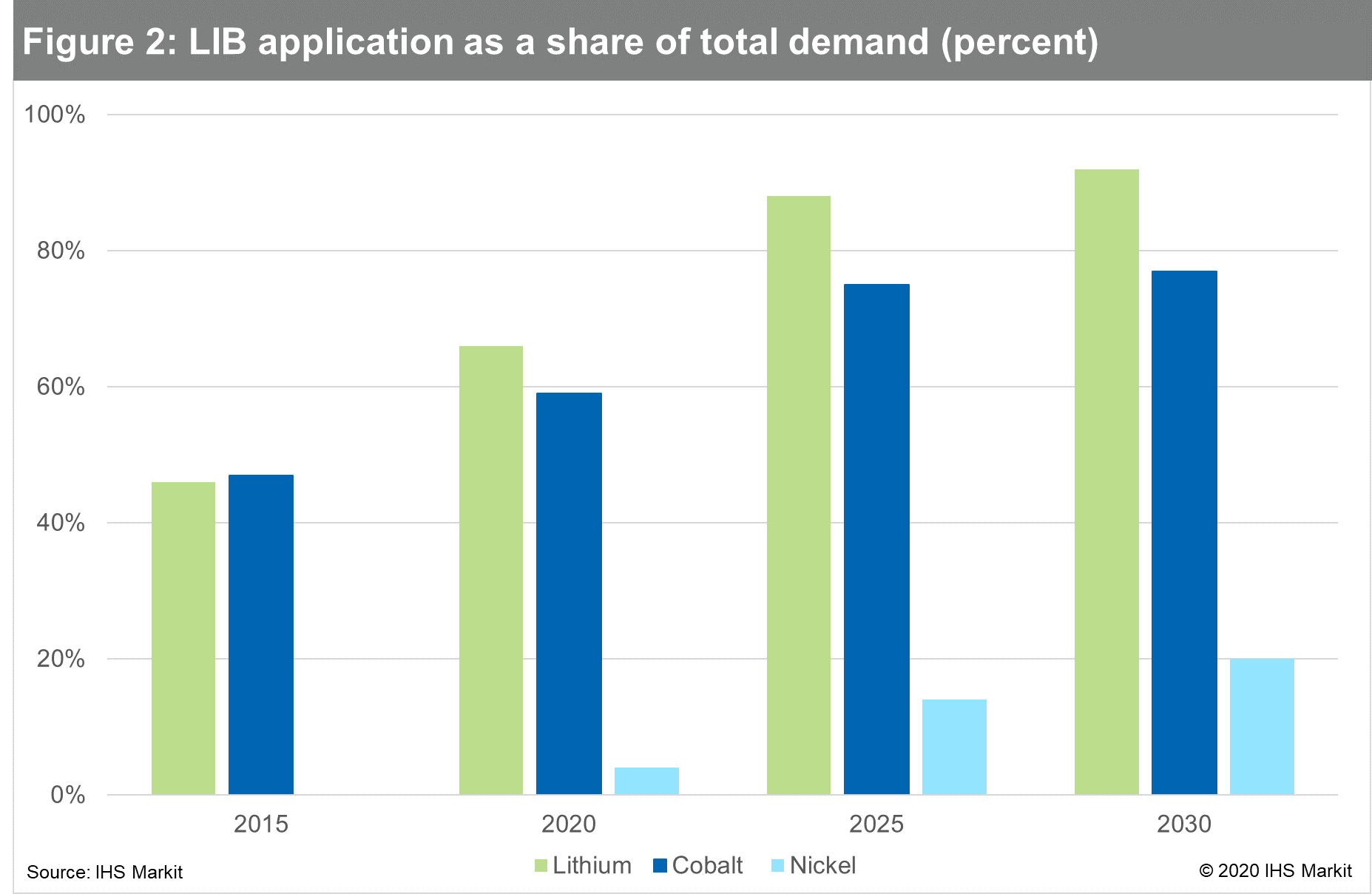
Sourcing of lithium raw materials and chemicals is
changing
Lithium is mined from both hard rock and underground
brine deposits. Lithium containing spodu- mene ore is mined from
open pits and typically concentrated by flotation methods on site.
Australia is the largest producer and exporter of spodumene
concentrate. Spodumene concentrate is converted into lithium
carbonate or lithium hydroxide, primarily in China. Most
brine-based lithium mining capacity is located in Chile and
Argentina. Brine is extracted from salars (salt-encrusted
depressions in the earth) and pumped into evaporation ponds, where
the power of the sun concentrates the brine. Lithium carbonate is
precipitated using soda ash or lime and can be further processed
into lithium hydroxide, which is required in new high-nickel
battery cathode chemistries.
The US was the biggest producer of lithium until 1997, utilizing
Nevada's brines and North Carolina's spodumene belt. For two
decades, brine-based production from Chile and Argentina dominated
the supply landscape, due to lower production costs. Emergence of
several Australian spodumene produc- ers over recent years has
changed this situation, and spodumene capacity now accounts for
more than two-thirds of lithium supply.
Other chemicals, electrolytes, and anodes play a
role
Electrolytes serve as a medium to transport lithium ions between
the cathode and anode in a battery cell. Typical liquid
electrolytes are mixtures of solvent (80%) and lithium salts and
additives (20%). The most widely electrolytes are used in the
majority of LIBs, and work is under way to develop ceramic (solid),
gel, and polymer electrolytes with even better electrochemical
performance. Electrolytes are currently produced in China, Japan,
and the US, but the development of Europe's own battery supply
chain has led to major investments in local producers, who could
commence production within the next five years.
LIB anodes are produced from spherical graphite. Graphite mining
and processing is linked to environ- mental pollution, so there is
less interest in expanding graphite-producing capacities globally.
China has large capacities in both the mining and processing
stages. Mozambique and Madagascar have ramped up capacity at new
mines over the last five years, with most output destined for China
for processing. Silicon, a more environmentally sustainable anode
material, could replace graphite in the future. Graphite and
silicon-blended anodes are already being used in combination with
some high-nickel NMC and NCA cathodes.
Supply should meet demand, but type and quality are
constantly evolving
Lithium, cobalt, and nickel production capacity should meet the
surge in demand. Established players are incrementally increasing
capacity, and many new producers are eager to gain their slice of
the electro- mobility cake. Cathode raw material suppliers will
have to remain flexible in their choice and quality of chemicals
offered so they can effectively supply this dynamic lithium-ion
battery revolution.