Published April 2024
Because of its unique properties, carbon dioxide is a versatile compound with many different applications in gaseous, liquid and solid states. It is used as a chemical building block (mainly for urea synthesis from ammonia), as an acidifier in beverage and water treatment applications, as a supercritical solvent (e.g., in enhanced oil recovery and caffeine extraction from coffee), as a shielding and inerting gas (e.g., in metalworking or food preservation) and as a chilling and cleaning agent while in a solid state (it sublimates from solid directly to gaseous state).
However, carbon dioxide is regarded as the leading climate issue for its role in accelerating global warming. CO2 is produced by the burning of fossil fuels (coal, natural gas, crude or refined oils) to generate electricity and heat or for transportation purposes; via the production of cement (the transformation from limestone or calcium carbonate to lime or calcium oxide releases CO2); and by various other industries. In 2023, mainland China was estimated to be the largest CO2-producing nation, followed by the United States and India.
Attempts are ongoing to limit and reduce CO2 emissions while collecting and sequestering carbon dioxide from the atmosphere. The chemical conversion and fixation of carbon dioxide, however, has limited applications for bulk use. Therefore, long-term geological storage, including usage for enhanced oil recovery, is probably the only practical way to reduce global carbon dioxide levels in the Earth’s atmosphere.
Although atmospheric carbon dioxide has been identified as a contributor to global warming, this is relevant primarily to industries that generate and release carbon dioxide into the atmosphere. The companies covered in this report recover and distribute by-product carbon dioxide or naturally occurring carbon dioxide but do not produce carbon dioxide.
The following chart shows world consumption of carbon dioxide:
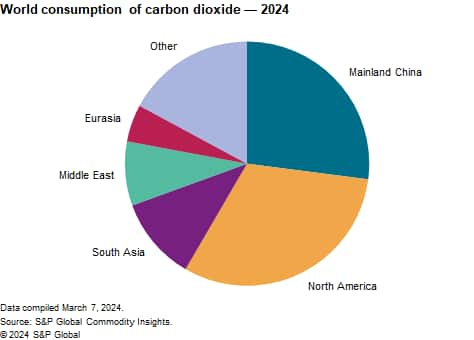
The carbon dioxide business is traditionally thought of as the recovery and distribution of liquid carbon dioxide, because this is the most commonly traded product. Liquid carbon dioxide is usually recovered as a gaseous byproduct of industrial operations, such as hydrogen production by the steam reforming of natural gas or the production of ethanol by fermentation. The gaseous carbon dioxide is liquefied for sale as a merchant product because liquid carbon dioxide can be transported more economically than gas. Many consumers also use carbon dioxide for the physical properties associated with it being a refrigerated liquid. Liquid carbon dioxide reaches end users through a network of highway tankers, resupply depots and railcars. Carbon dioxide is also traded as dry ice in the solid state, with its main end use being transport refrigeration. As a result of these circumstances, the carbon dioxide business is highly regional.
There is a substantial market for gaseous carbon dioxide for use in enhanced oil recovery. Another large use for gaseous carbon dioxide is on-site chemical manufacturing. For example, many ammonia manufacturers generate byproduct carbon dioxide and consume it at the same site for urea production.
The major issue in the carbon dioxide market is balancing regional supply and demand. Carbon dioxide sources may or may not exist where demand is greatest. In addition, chemical manufacturing operations that produce a gaseous carbon dioxide by-product run according to demand for the primary product, as opposed to demand for the byproduct carbon dioxide. For example, ammonia plants typically operate at full capacity in the fall and winter seasons in preparation for spring fertilizer requirements. Carbon dioxide demand, in contrast, tends to be highest during the warm summer months when ammonia plants may be closed for turnaround, so supplies are not often balanced with demand.
For more detailed information, see the table of contents, shown below.
S&P Global’s Chemical Economics Handbook – Carbon Dioxide is the comprehensive and trusted guide for anyone seeking information on this industry. This latest report details global and regional information, including
Key benefits
S&P Global’s Chemical Economics Handbook – Carbon Dioxide has been compiled using primary interviews with key suppliers, organizations and leading representatives from the industry in combination with S&P Global’s unparalleled access to upstream and downstream market intelligence, expert insights into industry dynamics, trade and economics.
This report can help you:
- Identify trends and driving forces influencing chemical markets
- Forecast and plan for future demand
- Understand the impact of competing materials
- Identify and evaluate potential customers and competitors
- Evaluate producers
- Track changing prices and trade movements
- Analyze the impact of feedstocks, regulations and other factors on chemical profitability

















